Get Latest Updates About Pharmacy Notes, Books and Many More
Aim
To determine the percentage purity of a given sample of Benzylpenicillin tablet.
Principle
Benzylpenicillin is assayed by the iodometric titration method. The titration is in which an equivalent amount of iodine is liberated to form potassium iodide by the sample and the liberated iodine is titrated against standard sodium thiosulphate solution. This type of indirect titration determination of compounds is called iodometric titration. In this titration, benzylpenicillin is first hydrolysed with sodium hydroxide solution converted to corresponding penicilloic acid (dicarboxylic acid). Then penicilloic acid is further treated with a mineral acid to form D-Penicillamine and benzyl penicillic acid. An obtained D-penicillamine is further oxidized quantitatively iodine to give disulphide, excess of iodine is
back titrated with 0.02M sodium thiosulphate, an equivalent amount of liberated iodine can be measured by titration with sodium thiosulphate using starch as an indicator, which is added near the endpoint as it get hydrolysed by hydrochloric acid and iodine gets trapped in the matrix of starch. Due to this, there is no continuous liberation of iodine. An endpoint is blue to apple green.
Reaction

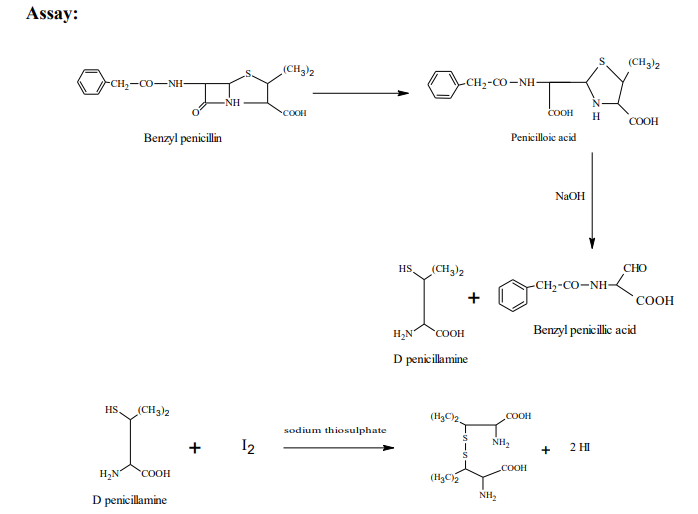
Procedure
Preparation of 0.02N Sodium thiosulphate
0.02N sodium thiosulphate (4.564 gm of sodium thiosulphate and 250 mg of sodium carbonate) dissolved in 100 ml of water then makeup to 1000ml with water.
Standardization of 0.02N sodium thiosulphate
Dissolved accurately weighed 0.2g of potassium bromate in 250ml of water taken in a conical flask. From this take 50 ml of the solution, add 2g of potassium iodide, 3ml of 2M HCl and titrate with sodium thiosulphate solution using starch as an indicator until the blue colour is discharged.
Each ml of 0.01N sodium thiosulphate= 0.002784g of KBr.
Assay of Benzyl penicillin
Weighed accurately about 0.1gm of the sodium salt of benzylpenicillin taken in a stoppered flask, dissolved in 10ml of water and dilute to 100ml. 10ml of the solution was transferred into an iodine flask, 5ml of 1N sodium hydroxide was added, allowed to stand for 20 minutes. Then freshly prepared buffer solution, 5ml of 1N hydrochloric acid and 25ml of excess of 0.02N iodine solution was added to the stoppered flask and kept aside for 20 minutes in a dark place. Excess iodine is titrated with 0.02N sodium thiosulphate using freshly prepared starch solution as an indicator. The endpoint is the discolouration of blue colour. To another 10ml of the initial solution add 20ml of the buffer solution,
allowed to stand for 20 minutes in the dark place and titrate with the same. The difference between the two titrations represents the volume of 0.02N iodine equivalent to the total amount of penicillin present in the given sample of benzylpenicillin.
Report
The molarity of 0.01M sodium thiosulphate = __________________
The percentage purity of given Benzyl penicillin tablet was found to be = __________________